OUR PIPELINE IS DESIGNED TO ADDRESS DISEASES WITH HIGH UNMET MEDICAL NEEDS
We paired our PLEX technology with drugs already approved by the U.S. FDA or innovative drug candidates
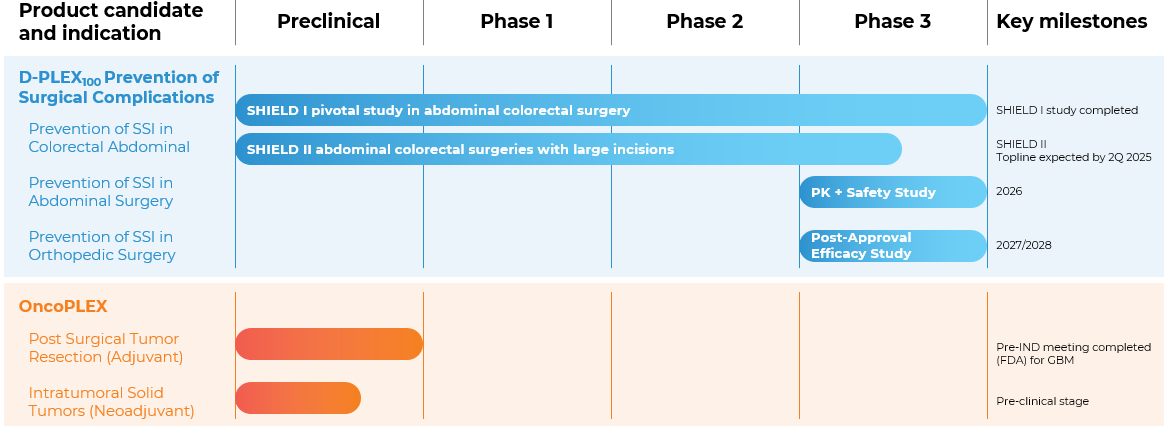
POLYPID'S PHASE 3 CLINICAL TRIALS:
SHIELD I ( NCT04233424 ) – Abdominal / Colorectal surgery
Efficacy and Safety of D-PLEX100 in the Prevention of Abdominal Surgery Incision Infection Post Colorectal Surgery.
Phase III, Prospective, Multinational, Multicenter, Randomized, Controlled, Two-arm, Double Blind Study to assess Efficacy and Safety of D-PLEX100 Administered Concomitantly with the Standard of Care (SoC), compared to a SoC treated control arm, in prevention of post abdominal surgery incision infection.
First patient in: July 2020
Last patient in: May 2022
https://www.clinicaltrials.gov/ct2/show/NCT04233424?term=PolyPid&draw=2&rank=4

SHIELD II (NCT04411199) – Abdominal/Colorectal surgery
Efficacy and Safety of D-PLEX100 in the Prevention of Abdominal Surgery Incision Infection Post Colorectal Surgery.
Phase III, Prospective, Multinational, Multicenter, Randomized, Controlled, Two-arm, Double Blind Study to assess Efficacy and Safety of D-PLEX100 Administered Concomitantly with the Standard of Care (SoC), compared to a SoC treated control arm, in prevention of post abdominal surgery incision infection.
First patient in: Q4 2020
Last patient in (expected): Q1 2025
Topline results (expected): Q2 2025
https://www.clinicaltrials.gov/ct2/show/NCT04411199?term=PolyPid&draw=2&rank=3
Expanded Access Policy
For new medical products to be legally approved for use, pharmaceutical companies are required to evaluate the safety and effectiveness of the medical product in clinical trials and submit trial results to regulatory agencies. To participate in a clinical trial, patients must meet certain criteria. For those who meet the criteria to join a clinical trial, participation offers the chance to contribute to medical research that may benefit many others.
At the same time, we understand that there are seriously ill patients who will not be eligible for our clinical trials and may have high risk to experience an infection post a surgical intervention. In these circumstances, regulators/health authorities may grant permission for us to provide a treating physician with an investigational drug pre-approval.
It’s important to remember that investigational drugs have not yet received regulatory approval; therefore, their potential risks and benefits are not yet established. Doctors and patients should consider all possible benefits and risks when seeking expanded access to an investigational drug.
Surgeons seeking pre-approval access for patients should submit their requests to clinical@polypid.com.
For more information please access PolyPid Expanded Access Policy.
