OVERVIEW
![]()
PolyPid has developed D-PLEX1000*, another product candidate from the PLEX-doxycycline family, for use in connection with orthopedic surgeries for the prevention of SSIs and support of bone recovery. Often, bone will not heal in the presence of infection.
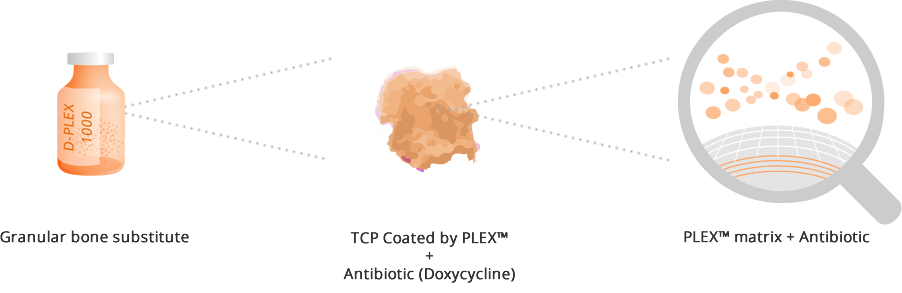
D-PLEX1000 is designed for filling bone voids, providing effective eradication of bone infection caused by doxycycline-sensitive microorganism around the implant by constant release of antibiotics.
D-PLEX1000 is comprised of synthetic, doxycycline eluting ß tri-calcium phosphate (ßTCP) granules.It is biocompatible and biodegradable and presented in a 10 gram vial that contains 65 mg of doxycycline hyclate.
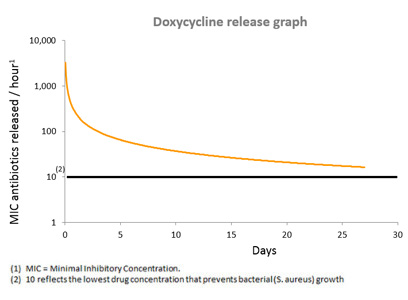
Upon hydration in the body, the PLEX™ matrix gradually degrades and allows the antibiotic entrapped between the layers to be released constantly between the granules, for a pre-designed period of at least three to four weeks.
It is serving as an osteoconductive bone scaffold supporting bone growth while gradually resorbing and replaced with bone during the healing process.
D-PLEX1000 is safe for immediate implantation into contaminated bones. As a result it has the potential to reduce the risk of infections, minimizing additional costly procedures and long hospitalizations, while enabling accelerated bone healing process.
VIDEO:
D-PLEX-1000
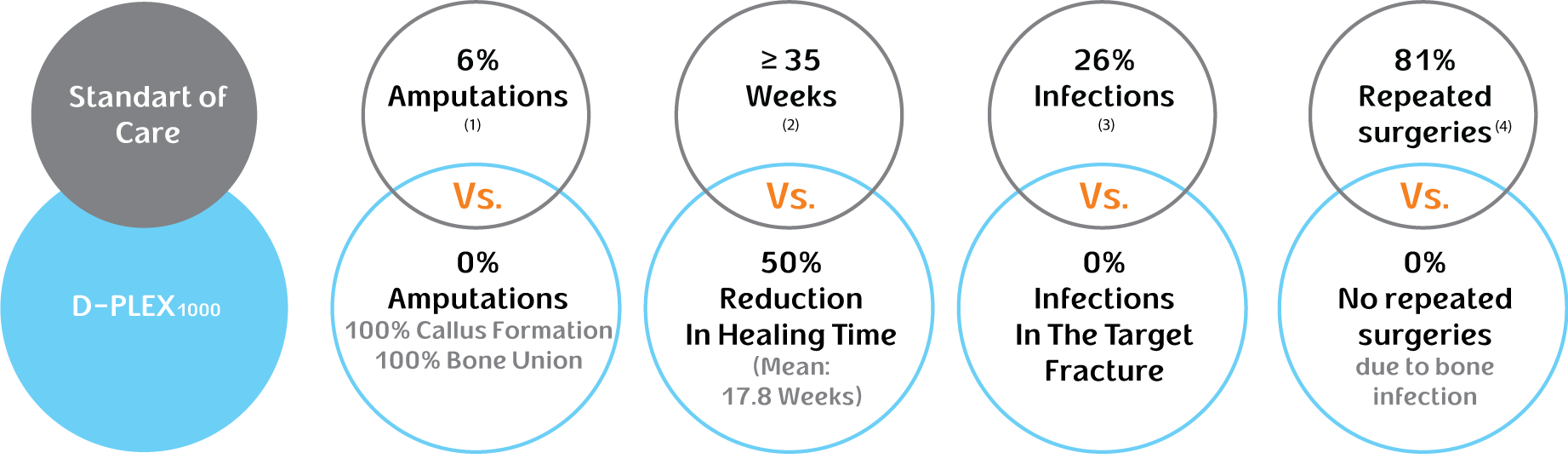
Based on data read out from our clinical trials of D-PLEX1000 thus far, none of the 34 patients treated with D-PLEX1000 developed infections in the target fracture, as compared with 26% of patients in the historical control group, and we observed a 50% reduction in bone healing time as compared to the historical control group.
D-PLEX1000 is not yet approved for sale by regulatory agencies.
*Formally known as BonyPid®-1000
FORMULATION
D-PLEX1000 formulation is well organized on a molecular level as a fine, sub-structure by self-assembly into PLEX™

LOCAL VS. SYSTEMIC DRUG DELIVERY
A low dose is sufficient to achieve a significant therapeutic effect
SYSTEMIC FORMULATION OF DOXYCYCLINE
Recommended oral dose for Doxycycline (tablets) - up to 200 mg/day (total of 6,000 mg)
![]()
D-PLEX1000 - DOXYCYCLINE
Estimated dose of Doxycycline hyclate in a single implantation - total of 65 mg
(Should be used in conjunction with systemic antibiotic)
D-PLEX1000 is designed to release a small antibiotic dose constantly for at least three to four weeks, with the overall accumulated dose being equal to about 1% of a 30 day oral antibiotic regimen.


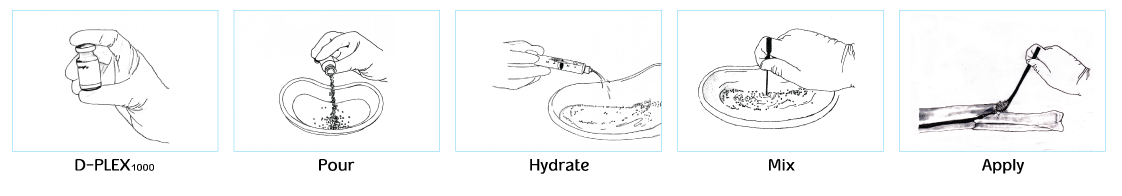
NO CHANGE IN THE METHOD OF USE IN SURGERY
Simple preparation and use
On top of Standard of Care
D-PLEX1000 is to be used in conjunction with standard of care in orthopedic surgeries.
CLINICAL TRIAL RESULTS SUMMARY
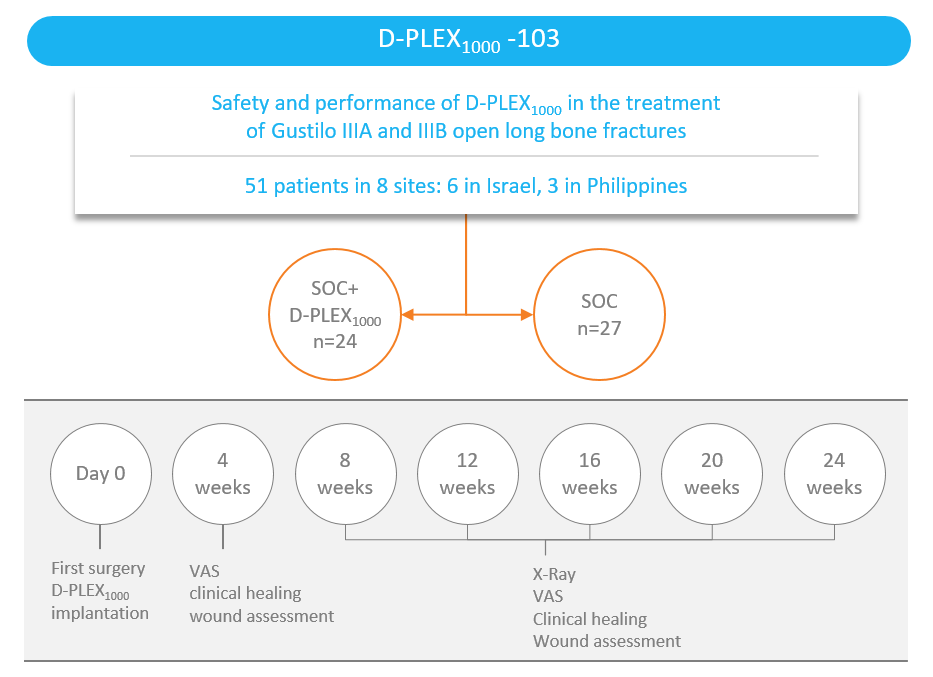
We have completed enrollment of a clinical trial of the safety and effectiveness of D-PLEX1000 for the treatment of open long bone fractures.
Study design
- The trial is a randomized and single blinded standard of care-controlled study in 4 patients with Gustilo* I and II open long bone fractures, as well as 47 patients with Gustilo IIIA and IIIB open long bone fractures, a severe clinical condition resulting from a traumatic high energy event where the bone is severely damaged and exposed and, therefore, assumed to be contaminated by environmental bacteria.
- The standard of care generally consists of administration of a systemic antibiotic before and after surgery, as well as irrigation and debridement.
- The objective of the study is to determine the safety and efficacy in bone healing of D-PLEX1000 in addition to the standard of care in traumatic open fracture patients over a period of six and 12 months, as compared to the standard of care alone.
- The primary performance endpoint is radiographic-assessed bone healing, as assessed by the presence of a callus in three out of four cortices, to be measured at the end of a 24-week follow-up period, based on independent blinded central radiographic evaluations of X-rays of the target fracture.
Results – Standard of Care vs. D-PLEX1000
We announced interim results from this trial of the first group of patients to reach the 16-week follow-up period. We observed that:
- In the 12 patients treated with D-PLEX1000 in addition to the standard of care, median time from surgery to the initiation of bone healing, as assessed by the presence of a callus in one out of four cortices, was reduced by approximately 31%, as compared to 12 patients in the standard of care only group.
- Median time from surgery to the primary endpoint, the presence of solid radiographic markers for bone healing, as assessed by the presence of a callus in three out of four cortices, was reduced by 20%, as compared to the standard of care only group.
- More than 30% of patients treated with the standard of care alone had not reached the primary endpoint, as compared to approximately 8% of patients treated with D-PLEX1000 and the standard of care.
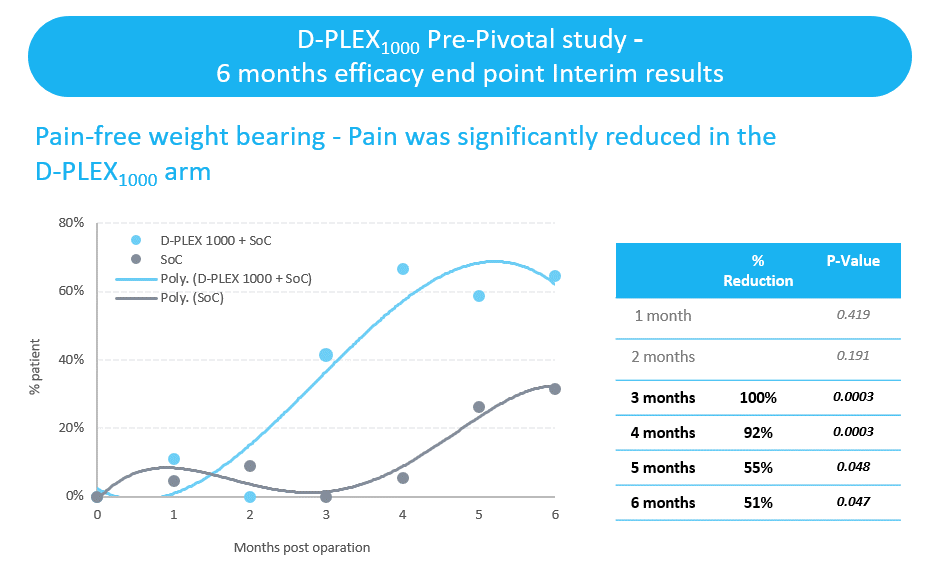
- Pain-free weight bearing was demonstrated in 63% of patients treated with D-PLEX1000 and the standard of care, as compared to none of the patients treated with the standard of care alone. We have announced interim results that indicated statistically significant reductions in self-assessments of pain using the Visual Analogue Scale twelve weeks after surgery.
- No product-related adverse events were reported.
We expect to report the full results of this trial in the second half of 2018.
* The Gustilo scale is a common classification for the severity of open fractures often used to guide the treatment regimen.
PILOT CLINICAL TRIAL RESULTS SUMMARY
Study design
16 patients with Gustilo type III A & B open tibia fractures, were immediately implanted with D-PLEX1000 during the first surgical intervention (Irrigation and Debridement), and stabilized by external fixation. Patients had periodic laboratory, bacteriology and radiology follow-ups for 6 months.
Results - Standard of Care vs. D-PLEX1000