LOCALIZED DRUG DELIVERY SYSTEM STUDIED FOR PREVENTION OF SSIs
Active Ingredient: Doxycycline (broad-spectrum antibiotic)
Release Duration: Prolonged effect of 30 days
Indication: Prevention of abdominal incisional surgical site infection (SSI)
Release profile: No burst, constant & linear release
Dosing: Varies by incision size 1vial < 10cm, 10cm < 2vials > 20cm, 3 vials >20cm
Negligible systemic exposure minimizes organ toxicity
High local concentration overcomes resistant bacteria and biofilm
Addresses area of great unmet need
INTUITIVE ADMINISTRATION ADAPTABLE TO MULTIPLE SURGERY TYPES
No additional cost or training needed
D-PLEX100 in bone tissue during open heart surgery
D-PLEX100 in Soft tissue during abdominal surgery
PHASE 3 SHIELD II - Prevention of Post Abdominal Surgery Incisional Infection
Design: multicenter, controlled, randomized double-arm study
Primary efficacy end-point: reduction in superficial and deep SSI, re-intervention and all-cause mortality within 30 days from index surgery
D-PLEX100 Showed Statistically Significant Reduction in the Primary Efficacy Endpoint
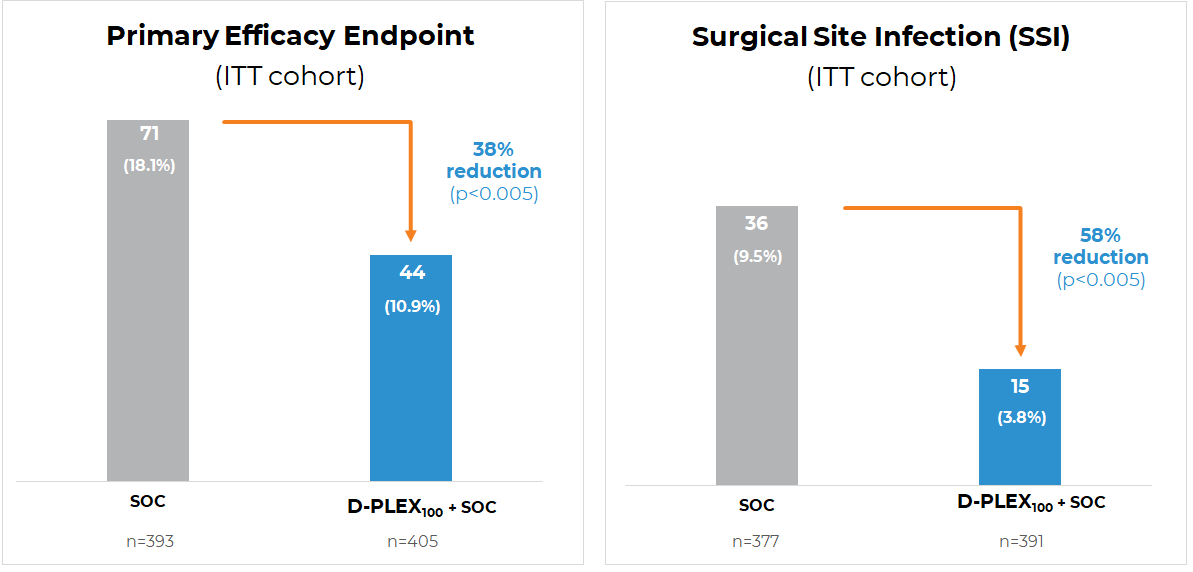
Standard of Care (SoC) includes IV antibiotic only / IV antibiotic with mechanical bowel prep / IV antibiotic with oral antibiotics combined with mechanical bowel
PHASE 2: PREVENTION OF STERNAL INFECTIONS POST CARDIAC SURGERY
Design: single-blinded and double-arm randomized study
Objective: safety and efficacy of D-PLEX100 + SoC (n=60) vs. SoC alone (n=21) in prevention of sternal infection post-cardiac surgery
Primary efficacy endpoint: the decrease of infection rate as measured by the proportion of subjects with at least one sternal infection within 90 days post-cardiac surgery
NO STERNAL WOUND INFECTION AND NO TREATMEN RELATED SERIOUS ADVERSE EVENT IN D-PLEX100 TREATED PATIENTS
|
Local Effects - Sternal Wound |
SoC |
D-Plex100 + SoC |
% Reduction |
|
Sternum Wound Infection rate (%) |
4.3% |
0% |
-4.3% |
|
Patient with sternum-wound discharge AE (%) |
26.1% |
8.6% |
-67% |
|
Duration of sternal discharge per patient (avg.) |
1.6 days |
0.7 days |
-56% |
|
Patient treated with i.v antibiotics directly duo to sternum-wound discharge AE (%) |
21.7% |
3.5% |
-84% |
|
General Effects |
SoC |
D-Plex100 + SoC |
% Reduction |
|
Re-hospitalization - % of patients |
38.1% |
26.7% |
-30% |
|
Re-hospitalization - duration / patient (avg.) |
7.6 days |
6.6 days |
-13% |
|
Overall re-hospitalization days per patient (avg.) |
2.9 days |
1.8 days |
-44% -1.1 days |
1 Adding vancomycin to perioperative prophylaxis decreases deep sternal wound infections in high-risk cardiac surgery patients. Reneike S. et al. European Journal of Cardio-Thoracic Surgery (2017) 2 Direct sternal administration of Vancomycin and Gentamicin during closure prevents wound infection. Andreas M. et al. Interactive CardioVascular and Thoracic Surgery (2017) 3Prevention of surgical site sternal infections in cardiac surgery: a two-centre prospective randomized controlled study. Schimmer C et al. European Journal of Cardio-Thoracic Surgery (2016) 4Surgical Site Infections Volume-Outcome Relationship and Year-to-Year Stability of Performance Rankings. Calderwood MS. et al. Med Care 2017;55: 79–85 .
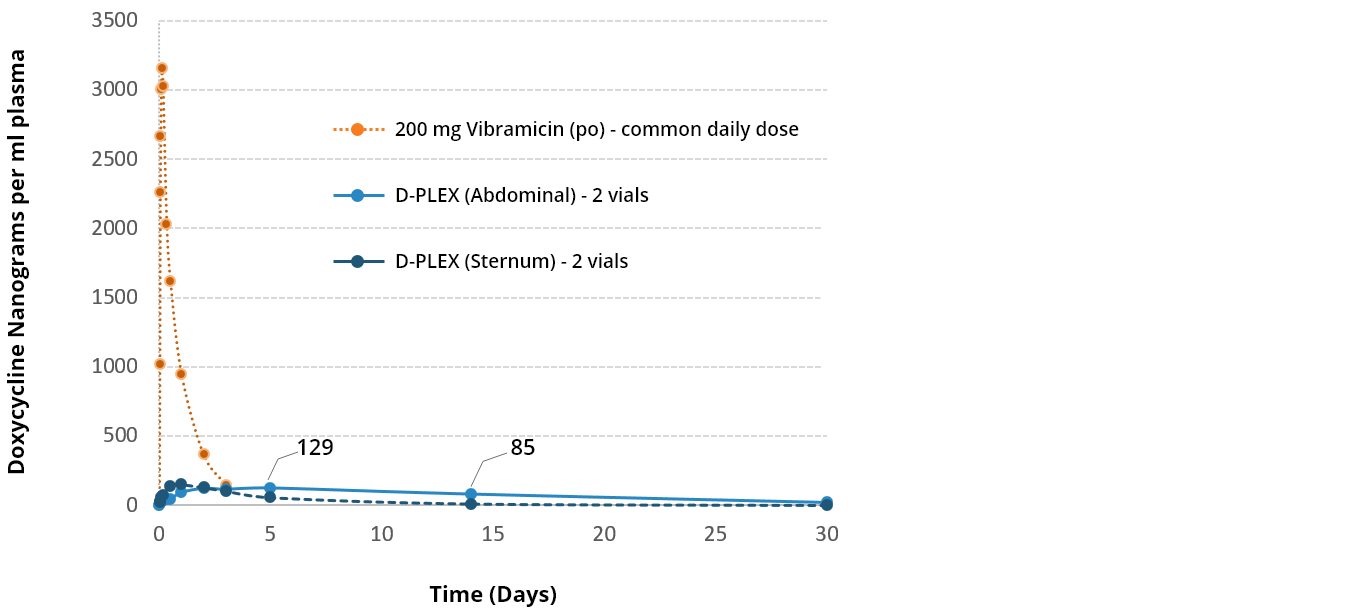
THE LOW SYSTEMIC EXPOSURE OF D-PLEX100 SUPPORTS HIGH SAFETY PROFILE
Comparison D-PLEX100 vs. Systemic administration of Doxycycline –
minimal systemic exposure – Sternum & Abdominal
THE BURDEN OF SITE SURGICAL INFECTION
Up to 30%
Estimated SSI rate of patients undergoing colorectal surgery1,27-11 days
Additional post-operative hospital days for patients with SSIs
20%
SSI rate of all health care-associated infection in US hospitals
2-11x
Increased risk of death for SSI patient (up to 40% mortality after deep sternal infection)
$11k-26k
Cost of treatment per infection directly attributable to SSIs
US / EU - $10bn / ~£11bn
Estimated SSI-related incremental annual hospital costs in the US and EU
Systemic antibiotics (IV or oral) used during the course of surgery, typically beginning an hour before surgery, are not effective to prevent all SSIs
The antibiotic penetration to the surgical wound during and post operation is significantly limited because of the surgical incision and the damage to blood vessels 1,2*
Systemic antibiotics may be limited in their ability to reach the target site due to surgical disruption of local blood supply.
